I am a PharmD and medical communications professional with a strong foundation in clinical research, drug safety, and regulatory writing, combined with a passion for strategic storytelling across scientific and commercial environments. Over the past five years, I have built a career that brings together science, communication, and brand development, producing high-quality and trustworthy content that connects with both expert and general audiences.
In my current role as a Senior Scientific Writer and Informed Consent Editor at Houston Methodist Academic Institute, I lead the development, editing, and regulatory review of informed consent forms, clinical protocols, and grant materials. I specialize in transforming complex research language into content that is clear, compliant, and easy for patients and participants to understand, while ensuring it meets institutional, federal, and global requirements.
Previously, I practiced as a Walgreens community pharmacist, where I verified and dispensed prescriptions with high accuracy; conducted prospective DUR to resolve interactions and duplications; counseled patients on new starts and chronic therapies; administered immunizations (influenza, COVID-19, Tdap, shingles); maintained vaccine inventory and controlled-substance compliance; resolved third-party/PA issues; and trained technicians and student interns. This frontline experience anchors my writing in real-world safety, workflow, and patient-education needs.
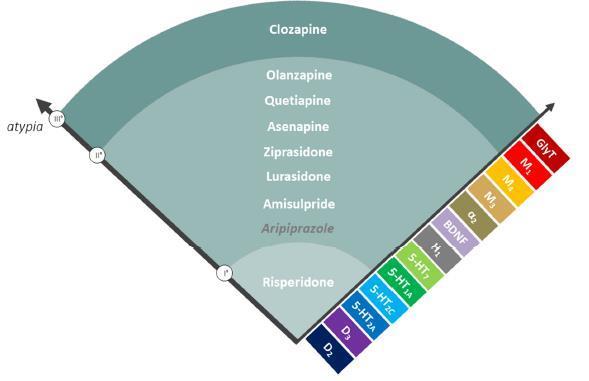
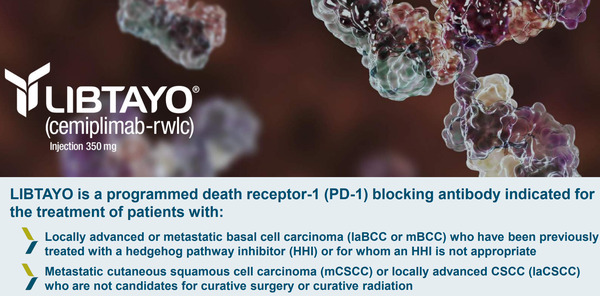
My background also includes experience in medical affairs, promotional marketing, and healthcare consulting. I have created peer-reviewed articles, conference abstracts, advisory board materials, and brand-centered messaging across multiple therapeutic areas, including psychiatry, oncology, infectious diseases, dermatology, and rare diseases. I regularly collaborate with key opinion leaders, interdisciplinary teams, and sponsors to produce content that is both informative and engaging, distributed across print, digital, and multimedia platforms.
Whether I am supporting clinical teams with regulatory documentation or developing promotional and educational resources, I bring a team-focused mindset, a focus on clarity, and a strong dedication to quality. My goal is to use science-based communication to promote understanding, build trust, and support better outcomes for healthcare professionals, research teams, and the patients they serve.
C.S. Lewis

I approach every project with a balance of clinical precision and narrative clarity, aiming to bridge the gap between science and understanding. Whether I am developing informed consent forms, peer reviewed manuscripts, or promotional materials, my process begins with listening. I take time to understand the needs of stakeholders, the context of the work, and the audience it is meant to reach.
My writing is guided by evidence based communication and shaped by years of experience in regulatory settings, medical affairs, and collaborative content development. I focus on turning technical, often complex information into content that is clear, compliant, and accessible. This applies whether the audience is a patient considering research participation or a physician reviewing therapeutic insights.
I believe in teamwork and thrive in collaborative environments. I have worked alongside clinical investigators, marketing teams, editorial staff, sponsors, and subject matter experts to make sure every piece of content is accurate, aligned, and meaningful. I bring structure to ambiguity, communicate clearly, and deliver work that is both timely and thoughtful.
My experience in freelance writing and consulting has strengthened my adaptability and awareness of audience needs. From internal education tools to patient summaries and brand focused campaigns, I adjust tone and style to meet the unique goals of each project.
At the core of how I work is a simple goal: to communicate in ways that build trust, improve understanding, and support better outcomes for healthcare teams, researchers, and the people they serve.